Can Taking Vitamin C Prevent The Flu
Abstract
Objective:
To investigate the relationship between the common cold and vitamin C supplementation.
Design:
A double-blind, 5-year randomized controlled trial.
Setting:
A village in Akita prefecture, one of the regions in Japan with the highest mortality from gastric cancer.
Subjects:
Participants in annual screening programs for circulatory diseases conducted under the National Health and Welfare Services Law for the Aged, and diagnosed as having atrophic gastritis. Of the 439 eligible subjects, 144 and 161 were assigned to receive 50 or 500 mg of vitamin C, respectively, after protocol amendment. During the supplementation phase, 61 dropped out, and 244 completed the trial.
Intervention:
Daily vitamin C supplementation of 50 mg (low-dose group) or 500 mg (high-dose group).
Results:
Total number of common colds (per 1000 person-months) was 21.3 and 17.1 for the low- and high-dose groups, respectively. After adjustment for several factors, the relative risks (95% confidence interval (CI)) of suffering from a common cold three or more times during the survey period was 0.34 (0.12–0.97) for the high-dose group. No apparent reduction was seen for the severity and duration of the common cold.
Conclusion:
A randomized, controlled 5-year trial suggests that vitamin C supplementation significantly reduces the frequency of the common cold but had no apparent effect on the duration or severity of the common cold. However, considering several limitations due to protocol amendment, the findings should be interpreted with caution.
Sponsorship:
This study was supported in part by Grants-in-Aid for Cancer Research and for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare of Japan.
Introduction
Many experimental studies have indicated that vitamin C affects the immune system. Vitamin C increases the proliferative responses of T lymphocytes and the production of interferon, and prevents defects in neutrophils (Hemila, 1996, 1997). However, the extent of the physiologic relevance of these effects on the susceptibility of human beings to infection is not well understood.
The effect of vitamin C on common cold incidence remains unclear. Numerous intervention studies have investigated the relationship between vitamin C supplementation and the incidence of common cold episodes. Six, large randomized trials of normally nourished subjects in Western countries, whose supplementation period ranged from 2 to 9 months, revealed no effect of vitamin C on common cold incidence (Anderson et al., 1972; Karlowski et al., 1975; Elwood et al., 1976; Ludvigsson et al., 1977; Pitt and Costrini, 1979; Briggs, 1984). However, studies of specific groups, such as subjects under heavy acute physical stress (Hemila, 1996) or British males with extremely low levels of vitamin C intake (Glazebrook and Thomson, 1942; Charleston and Clegg, 1972; Clegg and Macdonald, 1975; Baird et al., 1979), revealed significant reduction in common cold incidence with vitamin C supplementation. In addition, studies have consistently found reduced duration or severity of the common cold with vitamin C supplementation (Hemila and Douglas, 1999). Thus, studies to clarify both the preventive and the therapeutic effects of vitamin C on the common cold are needed. In this study, we examined the effect of 5-year vitamin C supplementation on common cold incidence, duration, and severity in a population-based, double-blind, randomized controlled trial.
Methods
Subjects
The initial aim of this study was to examine the effects of supplementation of β-carotene and vitamin C on the incidence of gastric cancer. Target subjects were men and women aged 40–69 years living in four municipalities of the Yokote Public Health Center District in the Akita prefecture, a region in Japan with one of the highest mortality rates from gastric cancer. The subjects were recruited through annual health checkup programs for circulatory diseases conducted during June–September 1995 by each municipality under the National Health and Welfare Services Law for the Aged. The rationale, design, and methods of the study have been described in detail elsewhere (Tsubono et al., 1997). Subjects with a diagnosis of chronic atrophic gastritis (defined as pepsinogen (PG) I<70 ng/ml and PG I/PG II ratio <3.0); no history of gastric cancer, gastric surgery, liver cancer, cirrhosis, or other cancers within the previous 5 years; no abnormal liver function (aspartate aminotransferase >100 IU/l, alanine aminotransferase >100 IU/l, or alkaline phosphatase >800 IU/l); no use of diet supplements containing β-carotene or vitamin C; and no expectation of moving outside the study area within 1 year were regarded as eligible. Written informed consent was obtained from each participant, and the Ethics Committee of National Cancer Center approved the protocol.
Study design and procedures
We first conducted a run-in phase, providing full doses of β-carotene (15 mg/day) and vitamin C (500 mg/day) to all participants for 4 weeks to identify and exclude at an early stage the subjects who either did not comply or showed side effects. All participants had started taking run-in capsules by December 1995. Participants were then randomized in a double-blind manner to one of four groups by using a 2 × 2 factorial design (0 or 15 mg/day β-carotene and 50 or 500 mg/day vitamin C) for the 5-year supplementation period. The assignment was based on simple randomization by using a table of random sampling numbers. The allocation of resources, participant enrollment, and group assignment were completed by one of the co-authors (YT). Participants were asked to visit community centers every 3 months, at which time public health nurses checked clinical symptoms and compliance by counting the number of unconsumed capsules, and then provided more capsules. At the baseline and fifth year of the study, participants also were asked to fill out a questionnaire inquiring about smoking habits, alcohol consumption, and medical history. A semiquantitative food frequency questionnaire with 108 items also was included in the main questionnaire.
In response to a US National Cancer Institute press release of January 18, 1996, indicating that two β-carotene trials had shown no benefit but had shown potential harm (Anonymous, 1996), we were obliged to amend the study protocol. Supplementation of β-carotene was stopped, but the prescription of vitamin C was continued for 5 years. The primary end point was altered from the 10-year cumulative incidence rate of gastric cancer to the 5-year change in serum levels of PGs, Helicobacter pylori infection, oxidative stress markers, and so on. On February 9 and 16, 1996, we invited the participants to community centers, explained in detail the results of the two US studies and the amendment of the study protocol, and collected the discontinued capsules from each participant. Signed consent was obtained again from individuals willing to remain in the study (N=244), and new capsules containing vitamin C only (50 or 500 mg/day) were provided. Although we estimated that a minimum of 1812 participants would be needed for the trial to detect a 40% reduction of the incidence of gastric cancer in the intervention group, the study area was restricted to the village from which participants had already been recruited; no new participants were recruited from the three other municipalities.
Vitamin C was expected to have a preventive or therapeutic effect on the common cold through effects on the immune system. In this report, we aim to evaluate the effect of vitamin C on the incidence, duration, and severity of the common cold.
Common cold survey
Episodes of the common cold were surveyed during the supplementation period (from years 2 to 5) and 1 year after the intervention was completed. Participants visited the community center every 3 months for new capsules and checkups by public health nurses who were blinded to the group assignment; the participants also were asked if they had caught a cold since the date of the previous visit. Thus, the information on the common cold covered all seasons. The first survey for the common cold was conducted during January–March 1997. All data were available to participants while they participated in the trial.
At 1 year after the supplementation phase was completed, a self-administered questionnaire was mailed to the participants inquiring about their history of diseases, endoscopy, and surgery, as well as episodes of the common cold during the previous year. The number of common colds (i.e., a cold so severe that the subject had to be in bed) during the period was determined by precoded answers: none, 1, 2, 3, 4, 5, and 6 or more. When subjects reported one or more episodes of the common cold, they were asked about the duration and severity of specific symptoms for the most severe cold. Duration of cough, nasal symptoms, sore throat, fever, headache, or muscular pain; days in bed; days absent from work; and total duration of the episode were ascertained. Severity of the cold was measured by using a typical scoring system introduced by Hemila (Hemila and Douglas, 1999; Table 1). For cough, nasal, throat, and systemic symptoms, a score of 0–3 was assigned according to the severity of each; then the total severity score was calculated. For those subjects who had not responded, two reminders with a new questionnaire were sent at 4 and 7 months after the first contact.
Full size table
Laboratory analysis
Fasting blood samples collected at baseline and after 5 years were analyzed for serum ascorbic acid levels, PG I, and PG II. All samples were stored at −70 to −85°C and were analyzed simultaneously after completion of the 5-year supplementation. All assays were conducted by persons who were blinded to the intervention assignment and the questionnaire data. The serum for ascorbic acid measurement was stabilized by the addition of meta-phosphoric acid. The level of serum ascorbic acid was analyzed fluorometrically (iodine oxidation and condensation with 1,2-phenylenediamine). Serum levels of PG I and PG II were measured by radioimmunoassay in a commercial laboratory (Dinabot, SRL Co. Ltd, Tokyo).
Statistical analysis
At each survey point, we calculated the incidence of the common cold (number of subjects; Figure 2) and total number of common colds (per 1000 person-months; Table 3). Person-months of follow-up were calculated from the date of the previous visit of the first common cold survey until the last survey. For those who withdrew from the trial, the date of dropout was treated as censoring. The Cox proportional hazards regression model was used to estimate the relative risks (RRs) of the common cold occurring one, two, or three or more times during the survey period. Age, sex, body mass index, smoking status, alcohol drinking, and the dietary intake of vitamin C, green or yellow vegetables, other vegetables, and fruits were included as covariates. Analysis of variance and analysis of covariance were conducted to investigate differences in the duration or severity of symptoms across supplementation groups. Age, sex, body mass index, past history of respiratory diseases, smoking status, alcohol drinking, and the dietary intake of vitamin C, green or yellow vegetables, other vegetables, and fruits were included as covariates. The analyses were conducted for subjects who completed the 5-year supplementation (completed group analysis; n=120 for the low-dose group and n=124 for the high-dose group), including those who dropped out after the protocol amendment (intention-to-treat analysis; n=144 for the low-dose group and n=161 for the high-dose group). Since the common cold survey began in the second year of the supplementation, the intention-to-treat cohort analyzed in this report consisted of 133 and 140 subjects in the low- and high-dose groups, respectively. Reported P-values are two-sided, and all statistical analyses were carried out by using the Statistical Analysis System (SAS) version 8.2 (SAS Institute, Inc., Cary, NC, USA).
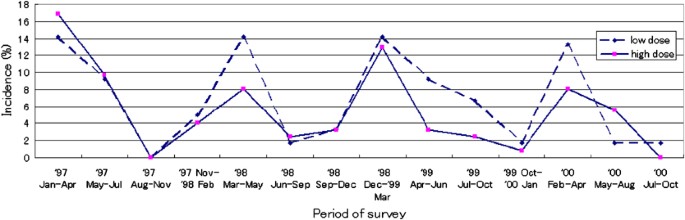
Common cold incidence, by supplementation group, during vitamin C supplementation.
Full size image
Full size table
Results
Of 1231 subjects screened through annual health checkup programs (June–September 1995), 1214 provided a blood sample for serum PG measurement (Figure 1). Among these subjects, 635 (52%) were diagnosed with chronic atrophic gastritis on the basis of serum PG level. A total of 33 people were ineligible since they did not meet the inclusion criteria. Of the remaining 602 eligible subjects, 439 (73%) consented to take part in the trial. Of the 439 subjects who initially participated in the study, 42 dropped out before the study was altered. Of the 397 remaining subjects, 305 (77%) consented to take part in the modified trial. After amendment of the protocol, 61 people withdrew for the following reasons: death (n=4), lost to follow-up (n=2), unknown (n=4), refusal (n=51). Refusal included other illness, minor side effect such as constipation or diarrhea (n=8; five for low-dose group, three for high-dose group), and heartburn (n=4 for high-dose group). A total of 244 subjects (80% of the subjects included in the modified trial) completed the 5-year supplementation. Table 2 shows baseline characteristics for the supplementation groups. No statistical difference except that for age was found between the low-dose (50 mg) and high-dose (500 mg) groups.
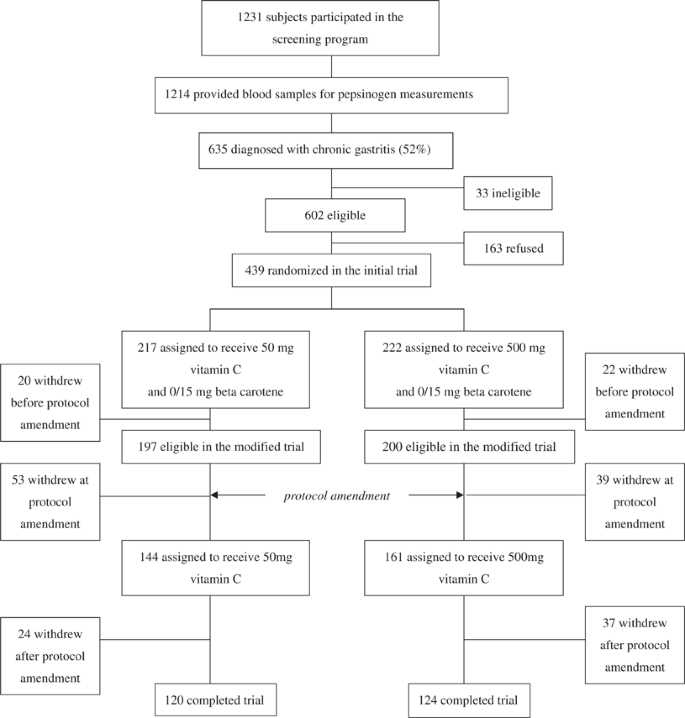
Study flow.
Full size image
Full size table
The incidence (%) of the common cold during the supplementation period for those who completed the supplementation is shown in Figure 2. For most of the survey period, the incidence of the common cold was higher in the low-dose than in the high-dose group. When the survey was conducted during April–June 1999, the incidence was 9.2% for the low-dose and 3.2% for high-dose group (P=0.05). The peaks of incidence (March–May 1998, December 1998–March 1999, February–April 2000) were as high as the incidence of the first survey (January–April 1997) for the low-dose group, whereas the peak incidence was consistently lower than that of the first survey for the high-dose group. The results were nearly identical for the intention-to-treat group.
On the basis of those subjects who completed the supplementation, total number of common colds (per 1000 person-months) during the supplementation was 21.3 for the low-dose group and 17.1 for the high-dose group (Table 3). Estimates of RRs were obtained for the common cold defined as one, two, or three or more times, with adjustment for potential confounding factors. When the common cold was defined as occurring three or more times during the survey period, an approximately 70% reduction in RR was observed (0.34, 95% CI: 0.12–0.97, P=0.04). The corresponding value for the common cold defined as occurring four or more times was 0.28 (95% CI: 0.06–1.28, P=0.10), for which only 10 events were observed. The results were essentially the same in the intention-to-treat groups.
The occurrence of common cold episodes also was surveyed after completion of the supplementation phase. Among those who completed the supplementation, 240 subjects received the self-administered questionnaire (four had died or moved). A total of 228 subjects (95%) returned their questionnaire; 146 (61%), 67 (28%), and 15 (6%) responded on first, second, or third contact, respectively. Among the 113 subjects in the low-dose and 115 in the high-dose group, 25 (25.7 %) and 20 (17.4 %), respectively, reported having caught cold during the previous year (P=0.13). The corresponding values for those who responded on first contact were 19 (24.7%) and 12 (17.4%), respectively (P=0.28).
Crude and adjusted means of duration (days) of common cold and specific symptoms for the two groups are shown in Table 4. After several factors were adjusted, the duration of cough, runny nose, sore throat, and total duration were longer in the high-dose group than in the low-dose group, although the difference was statistically significant only for runny nose. In contrast, duration of fever, headache, and muscular pain; days in bed; and days absent from work were longer in the low-dose group, although the differences were not statistically significant. When the analysis was repeated for those who responded on first contact, the observed difference in duration by group was more pronounced, although it still did not reach the level of statistical significance (data not shown).
Full size table
No statistically significant difference between groups was observed in the severity of the cold or each symptom (Table 5). The adjusted total severity score was 4.3 for the low-dose group and 4.2 for high-dose group (P=0.91). The results were similar when the analysis was restricted to those who responded at the first approach.
Full size table
Discussion
In this population-based, double-blind, randomized controlled trial, vitamin C supplementation was inversely related with common cold incidence, defined as a common cold episode occurring three or more times, while it had no effect on common cold duration or severity. Although vitamin C was supplemented for 5 years, the common cold survey period began in the second year and covered the period of 3½ years. Thus, a common cold episode occurring three or more times during the survey corresponds to about one time or more per year. However, our study has several disadvantages such as relatively small sample size, no clear definition of common cold, large drop out at protocol amendment, and lack of placebo arm. These points should be discussed before interpreting the present findings.
As mentioned above in the Method section, the sample size was calculated in accordance to the initial protocol that aimed to detect the reduction in gastric cancer rate. However, because of the protocol amendment, we restricted the subjects to one village and also 92 subjects dropped out from the trial at the time, which finally left 120 subjects for the low-dose group and 124 subjects for the high-dose group in the modified trial. Based on this sample size, standardized effect size of 0.30–0.40 can be detected by a setting of two-tailed test with α=0.05, 1−β=0.80. The observed standardized effect ranged from about 0.05 to 0.2 from our results in Tables 4 and 5. Consequently, the sample size in our study was considered to be not enough to detect the small effect of vitamin C on common cold duration or severity.
We did not set a clear definition of the common cold. On surveys done during the supplementation period, participants were asked if they had caught cold since the date of the previous visit. The definition of common cold fully depends on each participant's interpretation. Although the survey conducted after the supplementation phase limited the reporting of a common cold to those 'so severe that had to be in bed,' it still did not define what the common cold is. Thus, the present study possibly suffered some misclassification as regards disease status, which frequently masks the true association. There is no generally accepted definition of common cold. Except for a few studies (Karlowski et al., 1975; Ludvigsson et al., 1977; Pitt and Costrini, 1979), previous studies did not provide a clear definition of the common cold. Also, as in previous studies, the diagnosis was based on self-reports. Some studies have emphasized the use of seropositivity in the diagnosis (Cohen et al., 1993). However, a high proportion of common cold episodes that were not clinically detectable (i.e., false positive) were included in these studies (Takkouche et al., 2002).
Finally, only slightly more than half of the subjects randomized to the initial trial completed the supplementation; therefore, our findings should be interpreted cautiously. However, the protocol amendment (cessation of β-carotene supplementation and change in the end point from gastric cancer incidence to biomarkers) is not directly related to respiratory disease, including the common cold, or interest in vitamin C. In fact, there was no difference in baseline characteristics between the low-dose group (n=144) and high-dose group (n=161) who participated in the modified trial (Kim et al., 2004), which means that the randomization almost was maintained even though 134 subjects dropped out early in the study before and at the time of protocol amendment. Of the 305 subjects who participated in the modified trial, 244 (80%) subjects completed the trial. Moreover, as shown in Table 2, the baseline characteristics of the completed group were similar except for age; thus, it is unlikely that the dropout at the protocol amendment markedly reduced the scientific meaning of our results.
Although the role of vitamin C in the prevention and treatment of the common cold remains controversial, emerging evidence shows that vitamin C reduces mortality from heart disease or can reduce oxidative stress and lower the risk of age-related degenerative changes (Bsoul and Terezhalmy, 2004). Thus, although it is ideal to set a placebo arm, the vitamin C dosage of 50 mg, which corresponds to the recommended dietary allowance (Kurita, 1994) at that time, was set as low-dose group in our study. Furthermore, on the basis of our pilot study of 3-month supplementation of β-carotene and vitamin C (Sasaki et al., 2000), the serum level of ascorbic acid acted simultaneously for the placebo and 50-mg supplemented groups; the percent changes in serum ascorbic acid from baseline for the placebo and 50-mg groups were 31 and 23% at 1 month, 37 and 23% at 2 months, and 31 and 29% at 3 months, respectively. In comparison with previous studies, the supplementation amount of 500 mg per day for the high-dose group in our study does not seem large. According to a report (Levine et al., 1996), safe doses of vitamin C are less than 1000 mg daily, while bioavailability declines and the absorbed amount is largely excreted at single doses of 500 mg and higher. Thus, we set 500 mg as the dosage for the high-dose group. In fact, our previous report revealed a statistically significant difference in serum ascorbic acid level in the two groups after 5 years of supplementation (1.48 mg/dl for low-dose group, 1.73 mg/dl for high-dose group, P=0.0001) (Sasazuki et al., 2003). We did not assume that the dietary intake from food is nil. As shown in Table 2, the daily vitamin C intake from food is calculated as 150.8 and 149.9 mg among low-dose and high-dose group, respectively (P for difference=0.94). Also, the serum level of vitamin C did not differ between the group and this means that the background level of vitamin C is similar for low-dose and high-dose group. In our setting, we aimed to investigate the difference in effect of additional supplementation of vitamin C with 50 and 500 mg.
Potential bias is inevitable in any study. Although the subjects were randomized to each group and data were analyzed by considering several factors, the effect of unmeasured factors such as physical and psychologic stress may be important in evaluating the role of vitamin C on the common cold (Hemila, 1996; Takkouche et al., 2001). The findings in this study must be interpreted in light of possible sample selection bias, which may affect the ability to apply our results to other populations. Subjects of this trial were chosen on the basis of a diagnosis of atrophic gastritis by serum PG level (52% of those screened initially). In another village within the same district, the prevalence of atrophic gastritis was 55.4% among participants aged 40–59 years. Although the prevalence of atrophic gastritis was relatively higher in our study subjects than in other areas (Kabuto et al., 1993), our subjects do not represent a special group in Japan.
The initial trial started in September 1995, and all participants had started taking capsules by December 1995 and took them until the protocol amendment in February 1996. Thus, the period for which subjects took the initial capsule ranges from 2 to 5 months. On the basis of the results of our pilot study (Sasaki et al., 2000), no statistically significant interaction between 3-month supplementation of β-carotene and vitamin C was seen either for serum β-carotene or for ascorbic acid. In fact, further adjustment for initial β-carotene treatment did not have any influence on the effect of vitamin C on common cold; adjusted RR and 95% CI for suffering from common cold three or more times during the survey period was 0.35 (0.12–0.97) in high-dose group.
The effect of vitamin C supplementation on common cold incidence has been extensively studied. Pooled analysis of six major intervention trials of normally nourished subjects in Western countries showed that common cold incidence was not reduced in the vitamin C group, compared with the placebo group (pooled RR 0.99, 95% CI: 0.93–1.04) (Hemila, 1997). Our study differs from these previous studies in several ways. Our supplementation period was much longer than that of the six trials, which ranged from 2 to 9 months (Anderson et al., 1972; Karlowski et al., 1975; Elwood et al., 1976; Ludvigsson et al., 1977; Pitt and Costrini, 1979; Briggs, 1984). The long duration of supplementation may have resulted in the apparent reduction in incidence of the common cold. In fact, in a pilot supplementation study, the serum ascorbic acid level nearly reached a steady state and remained stable from 1 month onwards (Sasaki et al., 2000). In addition, the season during which a study is conducted could affect the apparent role of vitamin C in susceptibility to the common cold. Since common cold viruses are subject to frequent mutations, the type of strain might differ across years. In our study, all seasons were covered for several years, which may have minimized any distortion of the results.
As shown in Table 2, the dietary intake of vitamin C was about 150 mg/day for our study subjects, which is higher than that reported for the UK (30–60 mg/day) and the US (90–120 mg/day) (Hemila, 1997). Neither dietary intake of vitamin C nor serum level of ascorbic acid has been examined in most of the previous vitamin C trials. Thus, we could not compare our subjects' initial vitamin C status with other studies. As we had information of both dietary intake and serum level of vitamin C, we were able to analyze the data precisely.
In placebo-controlled trials, researchers have found consistently that the duration and severity of the common cold is reduced by regular vitamin C supplementation (Hemila, 1997). In these trials, the effect on duration of the cold was slight, but a more marked benefit was observed for severity, measured either directly or indirectly. According to the four largest trials, the relative difference of duration of colds or specific symptoms between intervention groups ranged from 3 to 7%, and the differences in duration of days confined to the house or absent from school (indirect measures of severity) were 21 and 14%, respectively (Anderson et al., 1972; Elwood et al., 1976; Ludvigsson et al., 1977; Pitt and Costrini, 1979). Our results are in line with the previous studies on the effect of vitamin C on common cold duration and severity, although our findings were not statistically significant.
A year after our subjects stopped vitamin C supplementation, the vitamin C group showed a limited immunity to the common cold. Complete elimination of vitamin C from the diet is reported to result in a zero level of ascorbate in serum within 35–40 days, in whole blood in about 80–90 days, and in white blood cells in 100–120 days (Goodhart and Shils, 1980). Although results did not differ between those who replied on first contact or later, the sample size in our study was small as mentioned above and the ability to detect a long-term effect of vitamin C supplementation may be limited.
In summary, our randomized, controlled 5-year trial suggests that vitamin C supplementation reduces the frequency of the common cold but has no apparent effect on its severity and duration. However, considering the several limitations due to the protocol amendment, cautiousness must be needed in interpreting the results.
References
-
Anderson TW, Reid DBW, Beaton GH (1972). Vitamin C and the common cold: a double-blind trial. Can Med Assoc J 107, 503–508.
CAS PubMed PubMed Central Google Scholar
-
Anonymous (1996). Beta Carotene and Vitamin A Halted in Lung Cancer Prevention Trial (press release). Washington, DC: US National Cancer Institute. January 18, 1996.
-
Baird IM, Hughes RE, Wilson HK, Davies JEW, Howard AN (1979). The effects of ascorbic acid and flavonoids on the occurrence of symptoms normally associated with the common cold. Am J Clin Nutr 32, 1686–1690.
CAS Article Google Scholar
-
Briggs M (1984). Vitamin C and infectious disease: a review of the literature and the results of a randomized, double-blind, prospective study over 8 years. In: Briggs MH (ed.), Recent Vitamin Research. Boca Raton, FL: CRC Press. pp. 39–82.
Google Scholar
-
Bsoul SA, Terezhalmy GT (2004). Vitamin C in health and disease. J Contemp Dent Pract 5, 1–13.
PubMed Google Scholar
-
Charleston SS, Clegg KM (1972). Ascorbic acid and the common cold. Lancet 1, 1401–1402.
CAS Article Google Scholar
-
Clegg KM, Macdonald JM (1975). L-ascorbic acid and D-isoascorbic acid in a common cold survey. Am J Clin Nutr 28, 973–976.
CAS Article Google Scholar
-
Cohen S, Tyrell DAJ, Russell MAH, Jarvis MJ, Smith AP (1993). Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health 83, 1277–1283.
CAS Article Google Scholar
-
Elwood PC, Lee HP, Leger AS, Baird IM, Howard AN (1976). A randomized controlled trial of vitamin C in the prevention and amelioration of the common cold. Br J Prev Soc Med 30, 193–196.
CAS PubMed PubMed Central Google Scholar
-
Glazebrook AJ, Thomson S (1942). The administration of vitamin C in a large institution and its effect on general health and resistance to infection. J Hygiene 42, 1–19.
CAS Article Google Scholar
-
Goodhart RS, Shils ME (1980). Modern Nutrition In Health and Disease 6th edn. Philadelphia: Lea & Febiger.
Google Scholar
-
Hemila H (1996). Vitamin C and common cold incidence: a review of studies with subjects under heavy physical stress. Int J Sports Med 17, 379–383.
CAS Article Google Scholar
-
Hemila H (1997). Vitamin C intake and susceptibility to the common cold. Br J Nutr 77, 59–72.
CAS Article Google Scholar
-
Hemila H, Douglas RM (1999). Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis 3, 756–761.
CAS PubMed Google Scholar
-
Kabuto M, Imai H, Tsugane S, Watanabe S (1993). Correlation between atrophic gastritis prevalence and gastric cancer mortality among middle-aged men in 5 years in Japan. J Epidemiol 3, 35–39.
Article Google Scholar
-
Karlowski TR, Chalmers TC, Frenkel LD, Kapikian AZ, Lewis TL, Lynch JM (1975). Ascorbic acid for the common cold: a prophylactic and therapeutic trial. JAMA 231, 1038–1042.
CAS Article Google Scholar
-
Kim MK, Sasaki S, Sasazuki S, Okubo S, Hayashi M, Tsugane S (2004). Long-term vitamin C supplementation has no markedly favourable effect on serum lipids in middle-aged Japanese subjects. Br J Nutr 91, 81–90.
CAS Article Google Scholar
-
Kurita H (1994). Ministry of Health, Welfare and Labor: Dietary recommended intakes. In: Recommended Dietary Allowance for Japanese 5th edn. Tokyo: Daiichi shuppan (in Japanese).
Google Scholar
-
Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR et al. (1996). Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93, 3704–3709.
CAS Article Google Scholar
-
Ludvigsson J, Hansson LO, Tibbling G (1977). Vitamin C as a preventive medicine against common colds in children. Scand J Inf Dis 9, 91–98.
CAS Article Google Scholar
-
Pitt HA, Costrini AM (1979). Vitamin C prophylaxis in marine recruits. JAMA 241, 908–911.
CAS Article Google Scholar
-
Sasaki S, Tsubono Y, Okubo S, Hayashi M, Kakizoe T, Tsugane S (2000). Effects of three-month oral supplementation of beta-carotene and vitamin C on serum concentrations of carotenoids and vitamins in middle-aged subjects: a pilot study for a randomized controlled trial to prevent gastric cancer in high-risk Japanese population. Jpn J Cancer Res 91, 1–7.
Article Google Scholar
-
Sasazuki S, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Kakizoe T et al. (2003). The effect of 5-year vitamin C supplementation on serum pepsinogen level and Helicobacter pylori infection. Cancer Sci 94, 378–382.
CAS Article Google Scholar
-
Takkouche B, Regueira C, Gestal-Otero JJ (2001). A cohort study of stress and the common cold. Epidemiology 11, 345–349.
Article Google Scholar
-
Takkouche B, Regueira-Mendez C, Garcia-Closas R, Figueiras A, Gestal-Otero JJ (2002). Intake of vitamin C and zinc and risk of common cold: a cohort study. Epidemiology 13, 38–44.
Article Google Scholar
-
Tsubono Y, Okubo S, Hayashi M, Kakizoe T, Tsugane S (1997). A randomized controlled trial for chemoprevention of gastric cancer in a high-risk Japanese population: study design, feasibility, and protocol modification. Jpn J Cancer Res 88, 344–349.
CAS Article Google Scholar
Download references
Acknowledgements
We express our appreciation to the staff at Hiraka General Hospital and the public health nurses at the Sannai village office for their support and assistance with the study.
Additional information
Guarantor: S Sasazuki.
Contributors: The study was designed and supervised by YT, SS and ST. SO, and MH coordinated the fieldwork and advised on the study design. SS analyzed the data, interpreted the current findings, and wrote the paper.
Rights and permissions
About this article
Cite this article
Sasazuki, S., Sasaki, S., Tsubono, Y. et al. Effect of vitamin C on common cold: randomized controlled trial. Eur J Clin Nutr 60, 9–17 (2006). https://doi.org/10.1038/sj.ejcn.1602261
Download citation
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/sj.ejcn.1602261
Keywords
- vitamin C
- common cold
Further reading
Can Taking Vitamin C Prevent The Flu
Source: https://www.nature.com/articles/1602261

0 komentar:
Posting Komentar